New strategy to harness red blood cell vesicles for gene therapies against cancer

A research led by Dr Minh Le, Assistant Professor in the Department of Biomedical Sciences at City University of Hong Kong (CityU) has developed a new strategy to generate large-scale amounts of extracellular vesicles (EVs) derived from red blood cells (RBCs) which can efficiently deliver gene therapies against cancer.
RNA drug therapies including small-interfering RNAs (siRNAs), antisense oligonucleotides (ASOs), and CRISPR–Cas9 genome editing guide RNAs (gRNAs) are emerging modalities for programmable therapies that target the diseased human genome with high specificity and great flexibility. However, common vehicles for RNA drug delivery, including viruses, lipid transfection reagents, and lipid nanoparticles, are usually immunogenic and/or cytotoxic. Thus a safe and effective strategy for the delivery of RNA drugs to most primary tissues and cancer cells, including leukemia cells and solid tumor cells, remains elusive.
Dr Le recalled that since there were some previous studies showing EVs, which are like the natural cellular postmen, are especially good drug carriers in blood cells, so she and the team thought of trying to use EVs to deliver ASOs. But here comes the question, “how do we get enough EVs for delivering the ASOs?” asked Dr Le.
She explained that stringent purification methods are required to produce highly pure and homogenous EVs, but they are time-consuming and not scalable. Moreover, the yield is so low that billions of cells are needed to get sufficient EVs, and such numbers of primary cells are usually not available. If immortalized cells, meaning those transformed with cancer genes for unlimited proliferation capacity, are used to derive EVs instead, there will be risk of horizontal gene transfer. It means that the oncogenic DNA and retrotransposon elements may transfer along with the RNA drugs. In fact, all nucleated cells present some level of risk for horizontal gene transfer, because it is not predictable a priori which cells already harbour dangerous DNA, and which do not.
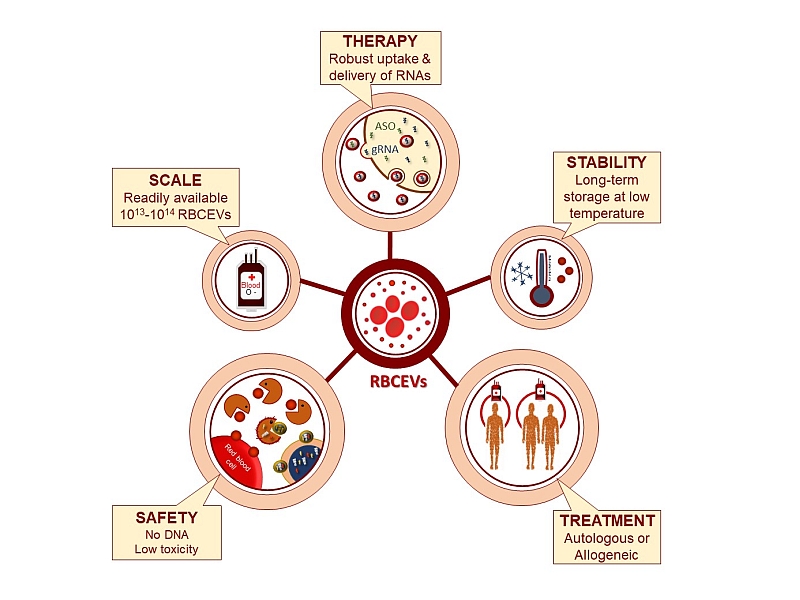
Hence Dr Le and her team decided to use human RBCs to produce EVs because RBCs lack both nuclear and mitochondrial DNA; they are the most abundant cell type in the body; and they can be obtained from any human subject readily.
In this research, Dr Le and her team have devised a new strategy to purify large-scale amounts of EVs from RBCs at low cost. Comparing with existing methods, the new strategy can generate 1000 times more of RBCEVs at only 1/4 of the cost. And they have shown that the RBCEVs can deliver the RNA drug to leukemia cells in a higher efficiency (with uptake efficiency of RBCEVs by leukemia cells up to 80%) and lower toxicity than some commonly used transfection reagents.
The research finding was published in Nature Communications under the title “Efficient RNA drugs delivery using red blood cell extracellular vesicles” in June.
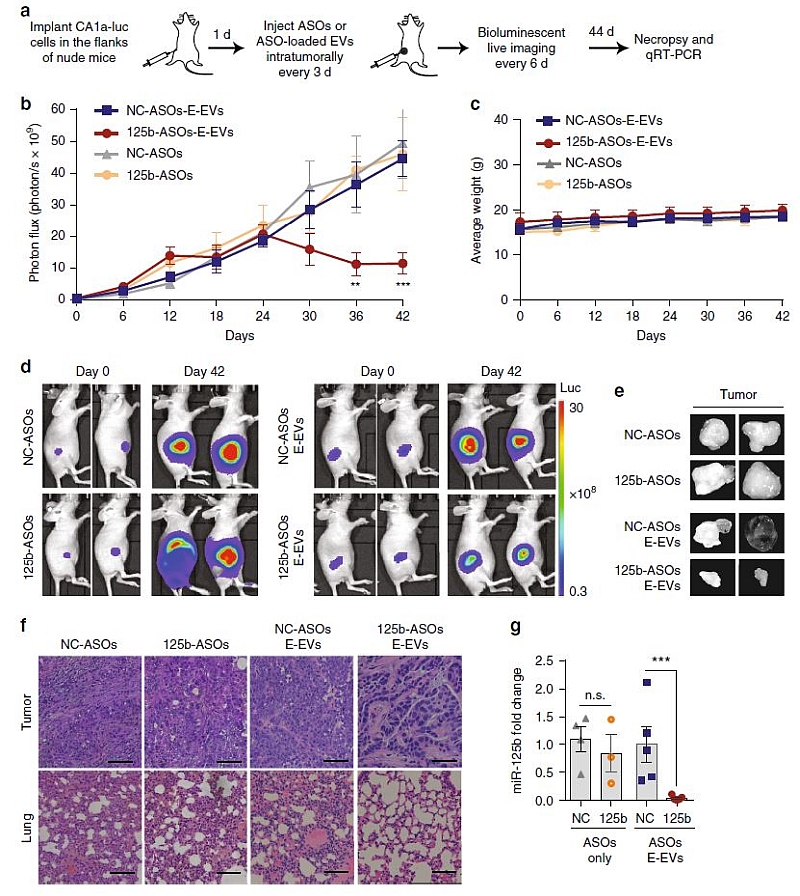
The team further investigated the therapeutic potential of RBCEVs in delivering ASOs that antagonizes miR-125b, which is a well-known oncogenic microRNA in leukemia cells, prostate cancer and breast cancer, and was well characterized by Dr Le in her previous work.
They demonstrated that the EVs loaded with anti-miR-125b ASOs was uptaken efficiently by both the breast cancer cells and leukemia cells in mice, and effectively suppressed the breast cancer and leukemia progression without any observable side effects.
The team also showed that the RBCEV platform could be used to deliver RNAs and DNAs for genome editing which could help to correct cancerous mutations, albeit with lower efficiency for large DNA plasmids and higher efficiency for RNAs.
Applications of the platform in genome editing are mainly developed by Dr. Shi Jiahai, who is an expert in novel RBC therapies and CRISPR Cas9, Assistant Professor in BMS, CityU and one of the co-authors of the paper. “This work is the result of a close collaboration between my lab and Dr. Shi’s lab. We also received great supports from our colleagues at CityU, collaborators from Queen Elizabeth hospital, Queen Mary hospital in Hong Kong and Genome Institute of Singapore. Only a team effort could help us develop the project very quickly in a short time. I am very thankful to all my students and collaborators for their hard work and keen support.”
Waqas Muhammad Usman, the first author of the article, said he was “excited” when the research results were positive. He and his teammates including Tin Chanh Pham, second author, and Boya Peng are research students of Dr Le. They all hoped that ultimately this technology can be translated into application in clinics. Tin added that their next direction would focus on modifying the RBC membrane to increase the specificity of RNA drug delivery.
Leading scientist in biomedical sciences, Harvey Lodish, who is founding member of the Whitehead Institute for Biomedical Research, Professor of Biology and Professor of Bioengineering at Massachusetts Institute of Technology, but was not involved in this research said that the finding shows “a new and important platform technology to deliver gene therapies or molecules to cells which can have many applications”. He was also the visiting professor at CityU.
He added that there are the two strengths of this research: it provides a procedure for making and purifying vesicles in a large amount, and a simple way of electroporation – loading nucleic acids (ie drugs for gene therapies) into those vesicles.
He believes there are still many steps, such clinical trials, before application in cancer therapy in human beings, but this finding is the very first step in potentially developing a very powerful therapy against cancer. And he thinks this is a perfect example of the kind of bio-technology that people would like to see developed in Hong Kong.
This project is funded by the CityU, Hong Kong Health and Medical Research Fund, Hong Kong Research Grants Council, National Natural Science Foundation of China, and Shenzhen Science and Technology Innovation Fund.