Cheap and efficient catalyst could boost renewable energy storage
Storing renewable energy as hydrogen could soon become much easier thanks to a new catalyst based on single atoms of platinum. The new catalyst, designed by researchers at City University Hong Kong (CityU) and tested by colleagues at Imperial College London, could be cheaply scaled up for mass use.
Co-author Professor Anthony Kucernak, from the Department of Chemistry at Imperial, said, "The UK Hydrogen Strategy sets out an ambition to reach 10GW of low-carbon hydrogen production capacity by 2030. To facilitate that goal, we need to ramp up the production of cheap, easy-to-produce and efficient hydrogen storage. The new electrocatalyst could be a major contributor to this, ultimately helping the U.K. meet its net-zero goals by 2050."
Renewable energy generation, from sources like wind and solar, is rapidly growing. However, some of the energy generated needs to be stored for when weather conditions are unfavorable for wind and sun. One promising way to do this is to save the energy in the form of hydrogen, which can be stored and transported for later use.
To do this, the renewable energy is used to split water molecules into hydrogen and oxygen, with the energy stored in the hydrogen atoms. This uses platinum catalysts to spur a reaction that splits the water molecule, which is called electrolysis. However, although platinum is an excellent catalyst for this reaction, it is expensive and rare, so minimizing its use is important to reduce system cost and limit platinum extraction.
Now, in a study published Sept. 13 in Nature, the team have designed and tested a catalyst that uses as little platinum as possible to produce an efficient but cost-effective platform for water splitting.
Lead researcher Professor Zhang Hua, from CityU, said, "Hydrogen generated by electrocatalytic water splitting is regarded as one of the most promising clean energies for replacing fossil fuels in the near future, reducing environmental pollution and the greenhouse effect."
Testing tools
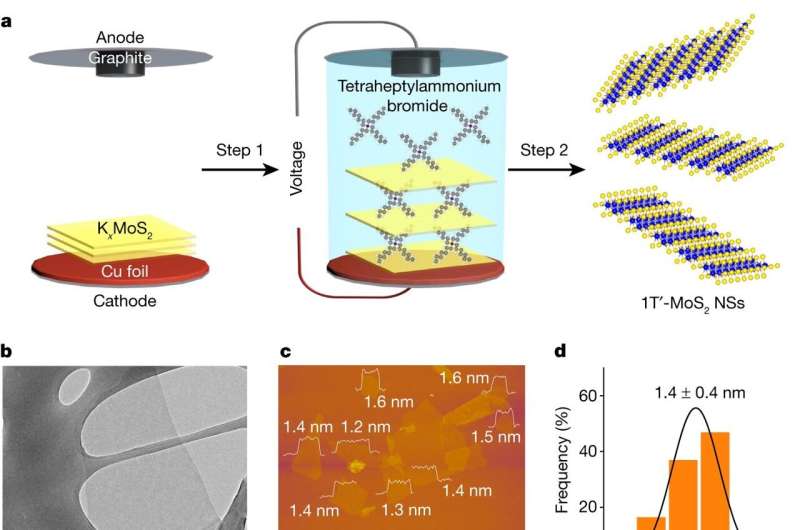
The team's innovation involves dispersing single atoms of platinum in a sheet of molybdenum sulfide (MoS2). This uses much less platinum than existing catalysts and even boosts the performance, as the platinum interacts with the molybdenum to improve the efficiency of the reaction.
Growing the thin catalysts on nanosheet supports allowed the CityU team to create high-purity materials. These were then characterized in Professor Kucernak's lab at Imperial, which has developed methods and models for determining how the catalyst operates.
The Imperial team has the tools for stringent testing because they have developed several technologies that are designed to make use of such catalysts. Professor Kucernak and colleagues have set up several companies based on these technologies, including RFC Power that specializes in hydrogen flow batteries, which could be improved by using the new single-atom platinum catalysts.
Using hydrogen
Once renewable energy is stored as hydrogen, to use it as electricity again it needs to be converted using fuel cells, which produce water vapor as a by-product of an oxygen-splitting reaction. Recently, Professor Kucernak and colleagues revealed a single-atom catalyst for this reaction that is based on iron, instead of platinum, which will also reduce the cost of this technology.
Bramble Energy, another spinout led by Professor Kucernak, will test this technology in their fuel cells. Both single-atoms catalysts—one helping turn renewable energy into hydrogen storage, and the other helping that energy be released as electricity later—therefore have the power to bring a hydrogen economy closer to reality.
More information: Zhenyu Shi et al, Phase-dependent growth of Pt on MoS2 for highly efficient H2 evolution, Nature (2023). DOI: 10.1038/s41586-023-06339-3



















